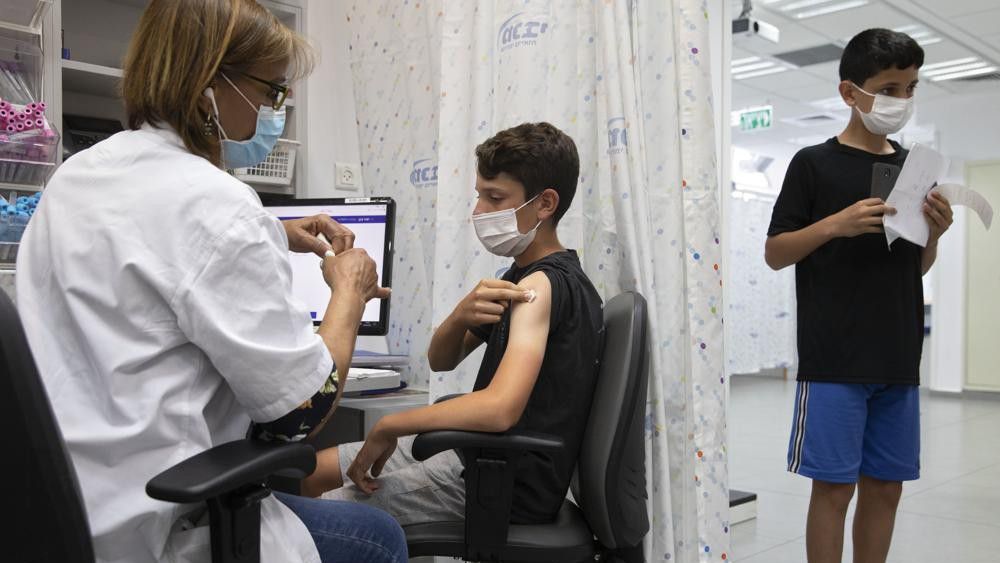
TEXAS — A Food and Drug Administration panel on Tuesday authorized emergency use of Pfizer’s pediatric COVID-19 vaccine for children ages 5-11.
The Texas Department of State Health Services on Monday said the state is already set to receive 1.3 million doses in the coming weeks.
Imelda Garcia, associate commissioner for DSHS’s Division for Laboratory and Infectious Disease Services, said the vaccine rollout will be different than it was last winter.
“We expect these children to be eligible to receive the COVID-19 vaccine very soon,” Garcia said.
According to Garcia, states were permitted to place vaccine orders prior to FDA emergency authorization and Centers for Disease Control and Prevention administration guidance.
The vaccines will arrive in Texas in three waves. Those vaccine will make their way to 814 providers in 120 counties in the first three waves of shipping, Garcia said.
Texas is home to approximately 2.9 million children between the ages of 5 and 11.
The first wave will include 404,100 doses and each subsequent wave will include 303,000 doses.
Pfizer’s two-dose coronavirus vaccine is currently being given to people as young as 12 in the U.S. Federal officials on Tuesday plan to discuss making smaller-dose versions available to the nation’s 28 million children between the ages of 5 and 11.
To help states and cities prepare, the CDC last week sent out a seven-page document with guidance on how to set up expanded vaccination programs.
For example, it notes pharmacies in every state can give COVID-19 shots to children, but it clarifies that only doses prepared and packaged specifically for children are to be used for those under 12.
It doesn’t speak to some thornier questions, however, such as how much school-based clinics should be relied on or whether kids should be required to get then shots as a condition of school attendance.
The Associated Press contributed to this report.